FGI Bulletin #21
YOU Have the Power…Until June 30, 2023
Users of the FGI Guidelines have the opportunity NOW to directly impact the text of the 2026 edition of the Guidelines for Design and Construction documents. Because the health care environment, practices, and technology continually evolve, we need those in the field to propose changes you think are needed to update and improve the Guidelines.
Is your Guidelines copy full of scribbles? Good! The time is NOW to convert those scribbles into proposals to revise the Guidelines before the last day of the proposal period—June 30, 2023. Once this window closes, no new revisions will be accepted for the 2026 edition of the Guidelines.
It’s easy to use FGI’s proposal platform. Register at https://fgiguidelines.net to browse the existing proposals, post opinions on them, filter for specific sections, and submit your own proposal on that pesky passage that tripped you up in the past.
We love your proposals and how they make the Guidelines better!
Interpretation Issued: Airborne Infection Isolation Patient Toilet Room Flooring
Should the flooring for the patient toilet room associated with an airborne infection isolation (AII) room be the same type required for the AII room itself? The 2022 Hospital Guidelines does not address this question, received from a Guidelines user, so FGI convened an interpretation committee to look into the matter.
“Airborne infection isolation room” is included on the list of room types in Section 2.1-7.2.3.1 (7)(a) (Floor and wall base assemblies) that must have a monolithic floor and wall base assembly with “an integral coved wall base that is carried up the wall a minimum of 6 inches and is tightly sealed to the wall.” The attached patient toilet room, however, is not mentioned. The interpretation committee members agreed the intent of the Guidelines is to require the toilet room associated with an AII room to have the same type of floor and wall base assembly as the AII room. They also agreed this should apply to the patient toilet room associated with a protective environment (PE) room. Their decision is consistent with inclusion of the anteroom to an AII or PE room on the list in Section 2.1-7.2.3.1 (7)(a). The goal is to support efficient cleaning and sterilization of all these spaces.
(Interpretations of Guidelines text are processed under the rules of the Health Guidelines Revision Committee (HGRC) and are considered an official position of the HGRC. This interpretation also applies to the 2018 edition of the Guidelines.)
Errata Updates
About Errata
Errata are errors in the published Guidelines books, whether editorial oversights or discrepancies revealed after publication. The errata sheets prepared for all Guidelines editions are crucial to users of the documents and are considered part of the official documents. These corrections should be applied as part of the standards by all users, including authorities having jurisdiction.
Users of the paperback version or those looking for a comprehensive list of errata (by edition) can find downloadable errata sheets on FGI’s website. Look for the yellow “Errata & Addenda” tab on the home page. Users of the digital version may rest assured that all corrections have been made to the digital view in any applicable section and edition on the digital site. (Note that the “page view” still shows the uncorrected print version text.) Users will be alerted to an erratum on any page containing a correction by the appearance of an errata tab at the top of the page.
Door Width
An erratum has been published to correct the minimum clear door width for geriatric patient bedrooms in a behavioral and mental health hospital in the 2022 edition of the Guidelines for Design and Construction of Hospitals as shown below:
2.5-2.5.7.1 Architectural details
(1) Doors. Door openings to patient bedrooms shall have a minimum clear width of 48 44.5 inches (1.22 meters).
Radiation Protection
When an erratum was published on 11/17/22 (see below), several other sections mentioning the 5-gauss exclusion zone in an MRI suite were missed; these have since been updated. Those sections are 2.2-3.5.5.5 (3), A2.2-3.5.5.7 (1), and 2.7-1.3.5.1 in the 2022 edition of the Guidelines for Design and Construction of Hospitals and sections 2.1-3.5.5.1 (2)(b) and (d), 2.1-3.5.5.5 (3), A2.1-3.5.5.7 (1)-c, and 2.13-1.3.5.1 in the 2022 edition of the Guidelines for Design and Construction of Outpatient Facilities. See the errata sheets for details.
2.2-3.5.5.1 Configuration of the MRI suite…
(1) Suites for MRI equipment with a static magnetic field of 5 gauss (0.5 millitesla) 9 gauss (0.9 millitesla) that is contained within the MRI scanner device shall conform with the manufacturer’s siting guidance.
(2) Suites for MRI equipment with a static magnetic field of 5 gauss (0.5 millitesla) 9 gauss (0.9 millitesla) that extends beyond the MRI scanner device shall meet the following requirements…
Reason for change: The Guidelines references IEC standard 60601-2-33 as the basis for a 5-gauss (0.5 mT) exclusion zone. That referenced standard now states the exclusion zone should be established at the 9-gauss (0.9 mT) volume. Identified in consultation with medical device standards bodies, the new IEC standard (9 gauss) is more permissive and requires a smaller exclusion volume than the prior 5-gauss value.
Clarification of Behavioral Health Crisis Unit Location
The erratum below reflects an agreed-upon clarification of the text by members of the 2022 Behavioral Health Crisis Unit Task Group after a possible misinterpretation was brought to their attention. This applies to the 2022 Hospital Guidelines.
*2.2-3.2 Behavioral Health Crisis Unit
…
*2.2-3.2.1.2 Location
…
(2) For renovations of existing hospital facilities, where it is not feasible for the unit to be in or readily accessible to the emergency department, the unit shall be permitted to be located elsewhere on the hospital campus
Location of Hand Scrub Facilities for Class 3 Imaging Rooms
To align with location language used throughout the Guidelines, the wording in the requirement below has changed from “directly outside” to “adjacent to.” “Directly outside” is not included in the location terminology defined in the Guidelines glossary; however, “adjacent” is defined as “located next to but not necessarily connected to the identified area or room.”
2022 Guidelines for Design and Construction of Hospitals
2.2-3.5.2 Imaging Rooms
…
2.2-3.5.2.3 Handwashing station or hand scrub facilities. Handwashing stations and hand scrub facilities shall comply with the requirements in sections 2.1-2.8.7 (Handwashing Station) and 2.1-2.8.6 (Hand Scrub Facilities) in addition to the requirements below.
(3) Hand scrub facilities shall be provided directly outside adjacent to the entrance to Class 3 imaging rooms.
2022 Guidelines for Design and Construction of Outpatient Facilities:
2.1-3.5.2 Imaging Rooms
…
2.1-3.5.2.3 Handwashing station or hand scrub facilities. Handwashing stations and hand scrub facilities shall comply with the requirements in sections 2.1-2.8.7 (Handwashing Station) and 2.1-2.8.6 (Hand Scrub Facilities) in addition to the requirements below.
(3) Hand scrub facilities shall be provided directly outside adjacent to the entrance to Class 3 imaging rooms.
Inclusion of Rehabilitation Unit Patient Room in Tables 2.1-2 and 2.1-3
The tables indicating locations for nurse call devices (Table 2.1-2) and requirements for oxygen, vacuum, medical air, WAGD, and instrument air outlets and inlets (Table 2.1-3) have been updated in the 2022, 2018, and 2014 editions of the Guidelines for Design and Construction of Hospitals to include requirements for rehabilitation unit patient rooms. This room type was erroneously omitted in both tables.
Numbering Corrections
2.8-3.4.3 Multiple-Patient Treatment Room
The following corrections in numbering apply to the 2022 edition of the Guidelines for Design and Construction of Outpatient Facilities.
2.8-3.4.3.1 General
…
(2) Combining bays to accommodate individuals of size shall be permitted. See Section 2.8-3.4.65 (Treatment Room for Individuals of Size) for more information.
2.8-3.4.8.2 Low-acuity patient treatment station…
…
(2) Patient treatment station features. See Section 2.1-3.2.3.2 2.8-3.4.4.2 (Patient care station features) for requirements.
Think You’ve Spotted an Error?
We appreciate hearing from Guidelines users who have questions about the content they use. This is often how errors are found. Write to us at info@fgiguidelines.org.
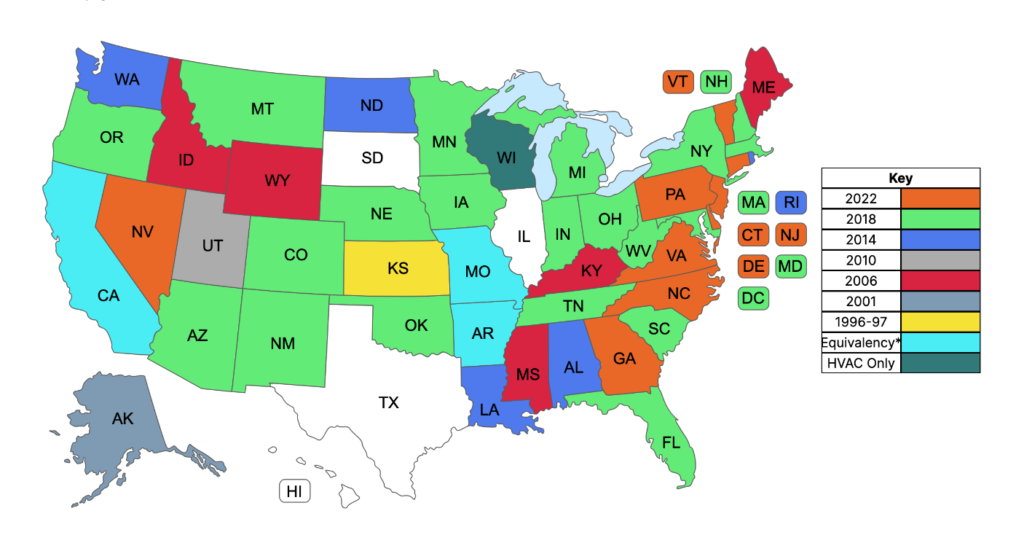
Adoption Map Updates
FGI provides a map on our website with state-specific information about adoption of the Guidelines documents. Different colors are used to identify the edition adopted by each state. In response to a user request, these colors were recently tweaked to make the map usable for most color-blind individuals.
As well, the 2022 edition has been added to the map, showing nine states that have adopted all or portions of the newest edition to regulate facility construction: Connecticut, Delaware, Georgia, Nevada, New Jersey, North Carolina, Pennsylvania, Vermont, and Virginia. Visit the adoption map and hover your cursor over each state to find details about how the Guidelines is being used
Alert
It has come to our attention that a small batch of 2022 Guidelines for Design and Construction of Hospitals documents contains printing errors. Examples of the errors include groups of missing, blank, and duplicate pages. These paperbacks were sold between May and December of 2022. If you purchased a copy of the 2022 Hospital Guideline during this time, please take a moment to look through it and let us know of any issues. Please use this link for reporting and requesting a replacement: https://wkf.ms/3kHK092